FLOXSAFE IV
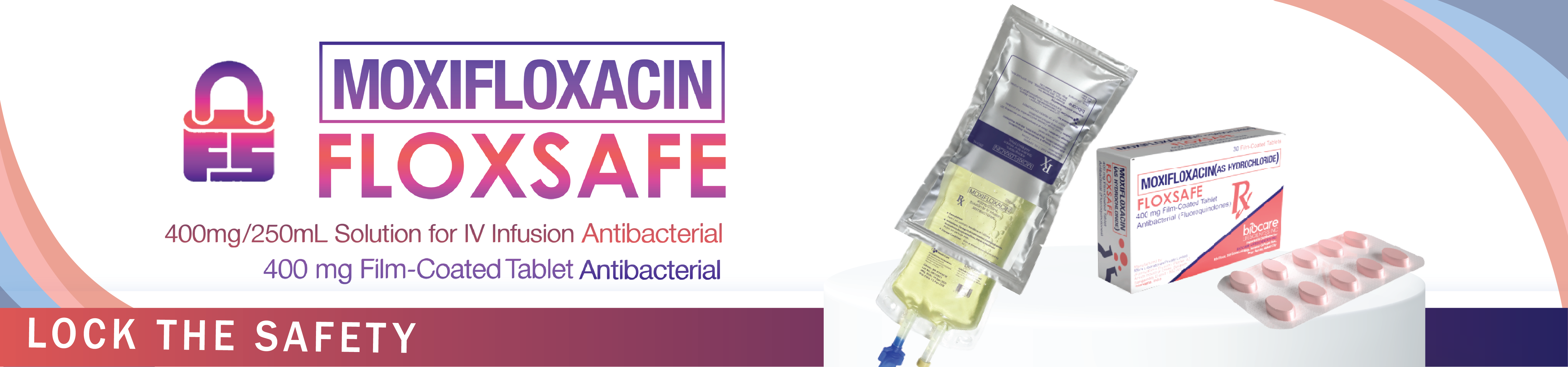
MOXIFLOXACIN
FLOXSAFE IV
400mg/250ml Solution for IV Infusion Antibacterial
Lock the safety!
- No left over self-collapsing bag
- Extra-Medication Port – Prevents drug contamination
- Non-PVC Flexible Bag – Prevents drug interaction
- Easy Handling – Compared to other IV Fluroquinolones preperation available in the market
INDICATIONS DAILY DOSE ROUTE OF ADMINISTRATION USUAL DURATION
Community Acquired Pnemonia (CAP) – 400mg IV 7-14 DAYS
Hospital Acruired Pnenomia (HAP) – 400mg IV/PO 7-14 DAYS
Ventilator Acquired Pneun
MOXIFLOXACIN
FLOXSAFE
400 mg Film-Coated Tablet
Antibacterial (Flouroquinoles)
- Easy switch from I.V. to Oral
- Approved for treating severe and life treatening infections.
- Bioequivalent to the innovator.
DON’T COMPROMISE YOUR SAFETY
Moxifloxacin [FloxSafe] is indicated in combination with other antituberculosis agents for the treatment of tuberculosis caused by Mycobacterium tuberculosis
Moxifloxacin [FloxSafe] is the indicated as a secondline antinycobacterial drug when use of first line drugs is not appropriate due to resistance or intolerance
WHO treatment guidelines for drug resistant tuberculosiss 2016 [update]
Therapautic Indications:
- AECOPD
- Acute Bacterial Sinusities
- Community Aquired Pneumonia
Also available in:
Mercury Drug
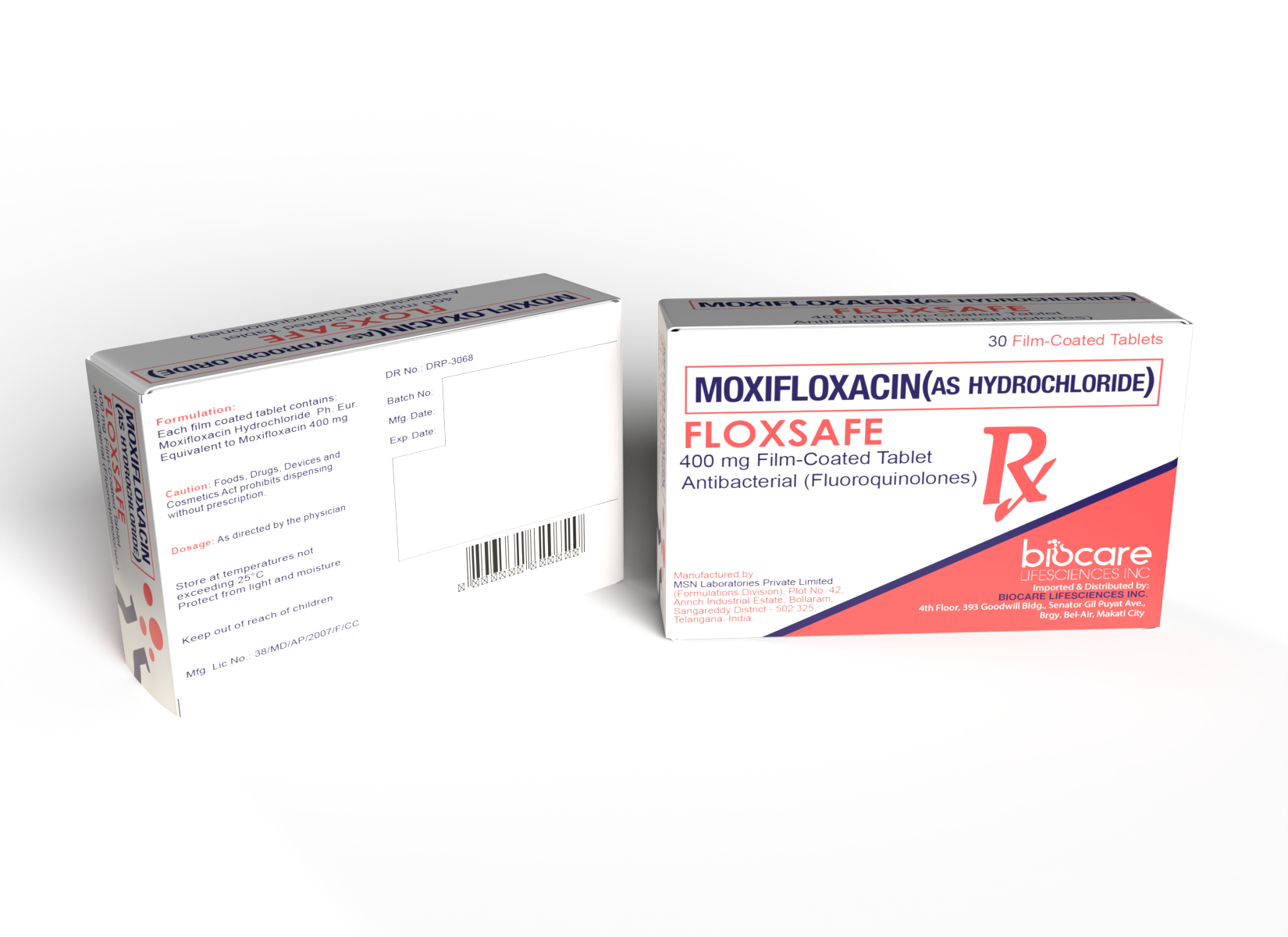
Formulation
Each film-coated tablet contains:
| Moxifloxacin Hydrochloride Ph. Eur. Equivalent to Moxifloxacin |
|---|
| 400mg |
View Complete Product Information
FLOXSAFE IV
400mg/250ml Solution for IV Infusion Antibacterial Lock the safety!- No left over self-collapsing bag
- Extra-Medication Port - Prevents drug contamination
- Non-PVC Flexible Bag - Prevents drug interaction
- Easy Handling - Compared to other IV Fluroquinolones preperation available in the market
- Easy switch from I.V. to Oral
- Approved for treating severe and life treatening infections.
- Bioequivalent to the innovator.
- AECOPD
- Acute Bacterial Sinusities
- Community Aquired Pneumonia


